Shaker Q. M. Nawasreh | Mohammad Hassan Ennab | Emad Rababah

9 -May-2019
Abstract
Treatment with radiolabeled somatostatin analogues is a future promising new tool in the management of patients with inoperable or metastasized neuroendocrine tumors, symptomatic improvement may occur with 177Lu-labelled somatostatin analogues that have been used for peptide receptor radionuclide therapy (PRRT), the results obtained with 177Lu-[DOTA0,Tyr3]octreotate (DOTATATE) are very encouraging in terms of tumor regression, dosimetry studies with 177Lu-DOTATATE as well as the limited side effects with additional cycles of 177Lu-DOTATATE suggest that more cycles of 177Lu-DOTATATE can be safely given. Also, if kidney-protective agents are used, the side effects of this therapy are few and mild and less than those from the use of 90Y-[DOTA0,Tyr3]octreotide (DOTATOC). Besides objective tumor responses, the median progression-free survival is more than 40 months, the patients’ self-assessed quality of life increases significantly after treatment with 177Lu-DOTATATE, I choosed this subject for my report because it is one of the rarest cancer treatment techniques used in medical centers all over the world, and it is rare to have research’s or proposed papers about it although the high importance and its costs, moreover the good therapeutic results gained through it, further more because it is the point where the work in nuclear medicine intersects with the rapidly developing radiotherapy techniques of today , it is the nuclear medicine way to treat tumors . Introduction In advanced and metastasized neuroendocrine tumors (NET), the use of surgery, external beam radiotherapy and chemotherapy as cytoreductive options is limited, The use of somatostatin analogues such as lanreotide ( C54H69N11O10S2 ) and octreotide(C49H66N10O10S2) not only reduces hormonal overproduction resulting in symptomatic relief, but has also been showed to increase time to tumor progression in prospective study in patients with functional midgut neuroendocrine tumors treated with long-acting octreotide. (9). It is where the Radio therapy intersects with the nuclear medicine, the field we call radionuclide therapy, it is a type of therapy where the radiation source is located in to the tumor structure chelating it with a protein that has a high ability to bind with the tumor cells specially, as other benefits are gained with the tumor irradiation, like inhibiting its growth an limiting its ability to secrete hormones or products. It was very beneficial to replace the external radiation sources with this type of radiation source as it can treat the very tiny molecular tumor structures without the need to do treatment plans or complex devices like linear accelerators or other radiotherapy equipment’s , and treat the very small size tumor structures which cannot sometimes distinguished through the medical imaging procedures (4). That is why the need to our study technique is becoming more important with time , and is in much times suggested instead of radio or chemotherapy techniques ,considering its therapeutic effects which are not related to the radioactive properties of the complex , but the biological effects related to the role of the bio-peptide attached to the tumor cells , which in most times acts to inhibit its growth and secretion , and so aids the radiotherapy side of the process .
Literature Review
Peptide receptor scintigraphy in humans started with the demonstration of somatostatin receptor-positive tumors in patients using a radio iodinated somatostatin analogue. Later, other radiolabeled somatostatin analogues were developed, and two of these subsequently became commercially available: [111In-DTPA0] octreotide (Octreoscan), and 99mTc-depreotide (Neotect). And then newer positron emission tomography radiopharmaceuticals have been developed.(7). In the early 1990s, treatment with radiolabelled somatostatin analogues started in patients with NETs. Peptide receptor radionuclide therapy (PRRT) started initially with [111In-DTPA0]octreotide with promising results such as symptomatic disease control, but partial remissions were rare. Lessons learned from these studies were that severe toxicities such as bone marrow suppression, and even myelodysplastic syndrome in patients treated with high dosages of ,(>3 Gy bone marrow radiation dose), as well as renal insufficiency and transient liver toxicity may occur.(7). The next generation of PRRT used a modified somatostatin analogue, [Tyr3]octreotide, with a higher affinity for the somatostatin receptor subtype-2. Thereby, a different chelator, DOTA instead of DTPA, was used in order to ensure a more stable binding of the intended β-emitting radionuclide 90Y. Using this compound (90Y-[DOTA0,Tyr3]octreotide (DOTATOC; Lastly 177Lu-based PRRT has been introduced into clinical practice.(7).
Chelator and peptide
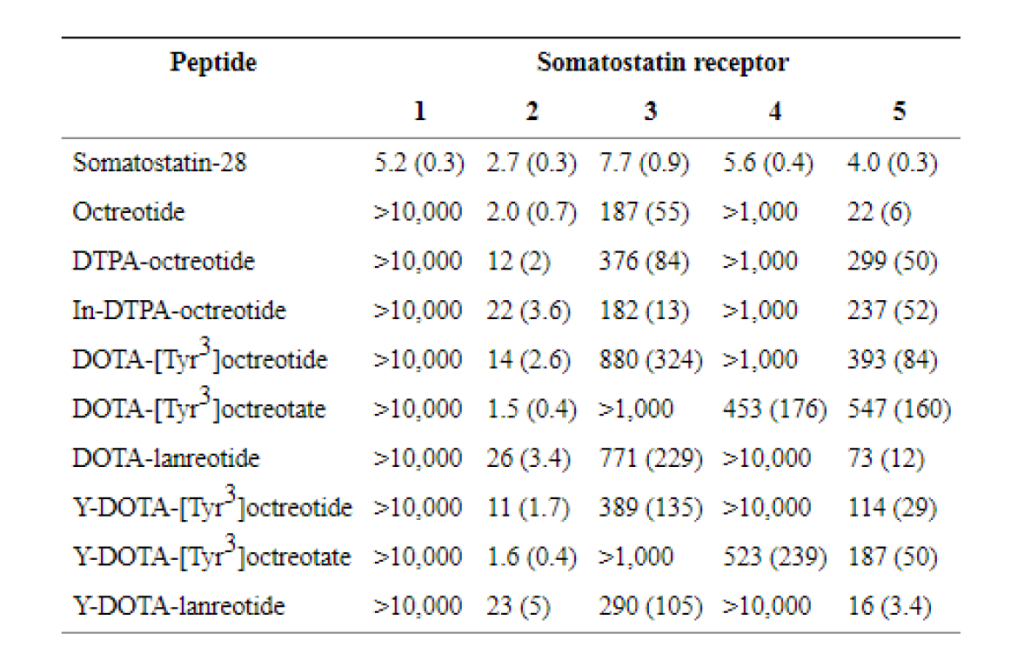
For the coupling of the radionuclide and the somatostatin analogue in PRRT, the chelator DOTA,is often used. Several studies on 177Lu-based PRRT have investigated the use of different somatostatin analogues, such as [DOTA0,Tyr3]octreotide (DOTATOC) , [DOTA0,Tyr3]octreotate (DOTATATE) and [DOTA0-1-Nal3]octreotide (DOTANOC). The general structure formula for the complex mostly include a between brackets protein in which in most times starts with the letters DOTA attached to some amino acid like tyrosine to act as a protein radioisotope chelator where the protein appears in the formula next out of the brackets, the total all formula is then named as a combination name for the previous mentioned two parts of the complex ,The somatostatin analogue [DTPA0,Tyr3]octreotate differs from [DTPA0,Tyr3]octreotide only in that the C-terminal threoninol is replaced with threonine, however, subtle changes in the structure of the chelator and the use of a different radionuclide or peptide affects the binding affinities for the different somatostatin receptor subtypes (Table1). In a comparison in patients, it was found that the uptake of radioactivity, expressed as a percentage of the injected dose of 177Lu-DOTATATE, was comparable with the use of 177Lu-DOTATOC in the kidneys, spleen and liver, but was three to four times higher in four out of five tumors. Therefore, 177Lu-DOTATATE has a potential advantage because of the higher absorbed doses that can be achieved in most tumors without increases in the doses to potentially dose-limiting organs. Also, in tumors in the same patients in a therapeutic setting, we found that the residence times are in favor of 177Lu-DOTATATE in comparison with 177Lu-DOTATOC by a factor of 2.1 (Fig. 1) .In contrast demonstrated no difference in tumor uptake of 111In-DOTATATE and 111In-DOTATOC, which is mostly referred to the ( In111 ) binding Effect which may disable some binding sites of the Peptide analogue , whereas 111In-DOTATOC showed a higher tumor-to-kidney absorbed dose ratio.
using 200 μg peptide with amino acid infusion, which corresponds exactly to the clinical therapeutic setting, Wehrmann et al compared the biodistribution of 177Lu-DOTATATE and 177Lu-DOTANOC in patients, and concluded that tumor uptake and absorbed doses were comparable for the two radio ligands, whereas whole-body retention was lower for 177Lu-DOTATATE, and therefore the authors advocated the therapeutic use of 177Lu-DOTATATE, because a lower whole-body retention potentially implies a lower bone marrow toxicity.(13).

Treatment protocols
Although most treatment protocols are much alike, minor differences do exist. All published studies on PRRT using 177Lu-based somatostatin analogues used diagnostic 111In-DTPA-octreotide (Octreoscan), 68Ga-DOTANOC or 68Ga-DOTATOC with sufficient tumor uptake as a patient inclusion criterion. Due to the need for kidney protection, most groups combined treatment with some form of amino acid infusion. The Rotterdam and Bad Berka groups use 2.5% L-lysine and 2.5% L-arginine in 1,000 ml, while the Basel group et al uses a 2,000-ml infusion of an amino acid solution comprising Ringer’s lactated Hartmann solution which is also known as sodium lactate solution and Hartmann’s solution, is a mixture of sodium chloride, sodium lactate, potassium chloride, and calcium chloride in water, It is used for replacing fluids and electrolytes in those who have low blood volume or low blood pressure. It is given by injection into a vein, Proteinsteril (B. Braun Medical), HEPA 8%, Mg 5-Sulfat to inhibit tubular reabsorption of the radio peptide. The Hartmann-HEPA solution typically contains 1% lysine, making the treatment protocol in terms of renal protection less effective as higher amounts of lysine lead to a greater reduction in renal uptake of radioactivity. This is striking, as Imhof group treated all patients from 1997 onwards with a confusion of 1,000 ml physiological saline containing 20.7 mg/ml of arginine and 20.0 mg/ml of lysine, The Milan group used 25 g of lysine in 1,000 ml saline infused over 4 h, followed by an additional 12.5 g of lysine in 500 ml saline over 3 h twice daily on days 2 and 3 after therapy. The recent report by the Gothenburg group et al does not mention the use of any form of kidney protection at all as many renal complications
5
appeared in their treated patients post therapy and it was striking to find more than 3% patients with renal failure in their treated population with PRRT.(14). The Bad Berka group studied eight patients with metastasized NET who received therapy initially with 177Lu-DOTANOC (mean injected activity 5,515 MBq, range 3,600–7,400 MBq), but continued the therapy with 177Lu-DOTATATE in three patients (injected activity not mentioned) as well as 61 patients who had one to four cycles of only 177Lu-DOTATATE (mean injected activity 5,534 MBq, range 2,500–7,400 MBq). Forrer et alused two cycles of 7,400 MBq 177Lu-DOTATOC with an 8-week interval in a study in 3 out of 28 patients with advanced paraganglioma and phaeochromocytoma and with predominantly small metastases (<2 cm), after a single cycle of 3,700 MBq/m290Y-DOTATOC.Other patients were treated with a single cycle of 7,400 MBq 177Lu-DOTATOC and those showing disease relapse after initial therapy with 7,400 MBq/m290Y-DOTATOC in two cycles. In a study by Kwekkeboom et al., patients who had not previously received PRRT were treated with 18.5 to 29.6 GBq of 177Lu-DOTATATE usually in four cycles, In a study by Bodei et al., patients were divided into two groups and received 3.7–5.18 GBq per cycle or 5.18–7.4 GBq per cycle with cumulative activities in the ranges 3.7–29.2 GBq and 5.55–28.9 GBq, respectively the overall appearance of the previous mentioned studies showed a better tumor response and symptomatic improvements with patients after treatment with Lu177 DOTATAT than any other treatment with other radioisotopes or somatostatin analogues shown in the figure 2.


Therapy response
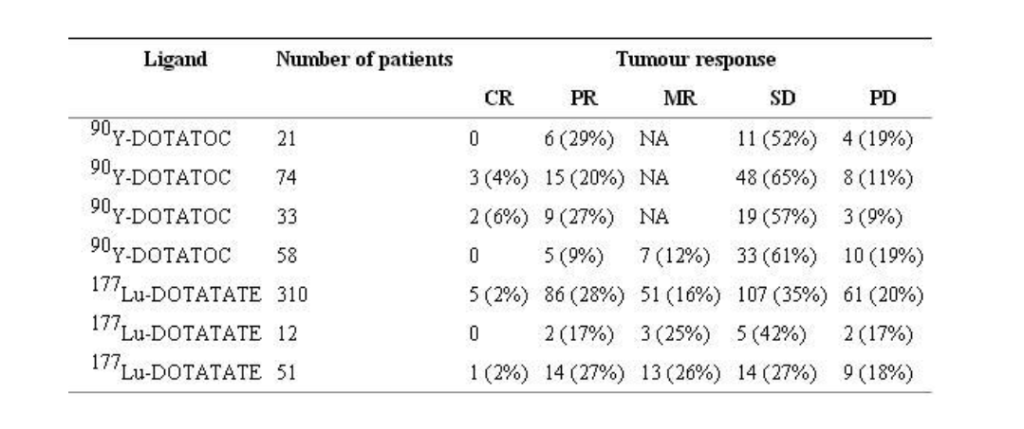
Kwekkeboom et al analyzed responses to 177Lu-DOTATATE treatment according to tumors type at 3 months after the last therapy cycle in 310 patients. Patients were treated up to an intended cumulative activity of 22.2–29.6 GBq (600–800 mCi). The overall objective tumor response rate including complete remission (CR), PR and minor response (MR) was 46% (Table 2) (Fig. 2). Prognostic factors for predicting tumors remission (CR, PR or MR) as the treatment outcome were high on diagnostic Octreoscan imaging. A small percentage of patients who had either stable disease (SD) or MR at their first two evaluations after therapy, i.e. 6 and 12 weeks after the last treatment cycle, had a further improvement in categorized tumor response at 6 months and 12 months, occurring in 4% of patients and 5% of patients, respectively. Three of four patients with clinically nonfunctioning neuroendocrine pancreatic tumors that were judged inoperable before treatment with 177Lu-DOTATATE, and who had a PR, were successfully operated on 6–12 months after their last treatment, but the fourth died of postoperative complications. In a small group of 21 patients treated with 177Lu-DOTATATE by Garkavij et al. 12 were evaluated for 7 objective response using RECIST criteria. PR was found in two patients, MR in three and SD in five. In the last study reported by Bodei et al. 1 patient had CR, 14 had PR, 14 had MR, 14 had SD, and 9 had progressive disease (PD).
Another study evaluated the quality of life (QoL) in our first 50 Dutch patients with metastatic somatostatin receptor-positive GEP tumors treated with 177Lu-DOTATATE . The patients completed the European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire C30 before therapy and during the follow-up visit 6 weeks after the last cycle. A significant improvement in the global health status/QoL scale was observed after therapy with 177Lu-DOTATATE. Furthermore, significant improvement was observed in emotional and social function scales. The symptom scores for fatigue, insomnia and pain decreased significantly. Patients with proven tumors regression most frequently had an improvement in QoL domains. However, because of the lack of a control group in this study, some placebo effect cannot be ruled out completely. This was also confirmed in a later study in 265 patients by Khan et al. who demonstrated not only an improvement in QoL, but also in Karnofsky performance score. Furthermore, no decrease in QoL was found in patients without symptoms prior to PRRT. Avery important side of the PRRT is the psychological effects associated with the suggestion of this type of therapy to patients as when patients knows that conventional chemotherapy with its will known side effects will be avoided and replaced by this therapy type the mostly show hopeful feeling as the questioner performed by European Organization for the Research and Treatment of which showed over 42% psychological improvement in patient just after suggestion of chemo or conventional radiotherapy .
Options to improve PRRT
From animal experiments it can be inferred that 90Y-labelled somatostatin analogues may be more effective in larger tumors, whereas 177Lu-labelled somatostatin analogues may be more effective in smaller tumors, but their combination may be even more effective. Therefore, apart from comparisons between radiolabeled octreotate and octreotide, and between somatostatin analogues labelled with 90Y and those labelled with 177Lu, PRRT with combinations of 90Y- and 177Lu-labelled analogues should also be evaluated. Future directions to improve this therapy may also include the use of radio sensitizing chemotherapeutic agents. Chemo sensitization with 5-fluorouracil (5-FU) in combination with 90Y-labelled antibody radio immunotherapy is feasible and safe . Also, chemo sensitization with 5-FU combined with [111In-DTPA]octreotide treatment resulted in symptomatic response in 71% of patients with NETs , whereas other studies using only [111In-DTPA]octreotide treatment have showed such responses in lower percentages . Numerous trials on the effects of combined chemotherapy and (fractionated) external beam radiotherapy have been performed. More recent trials used the prodrug of 5-FU, capecitabine, which has the advantage of oral administration. Also with the combination of radiotherapy and capecitabine, increased efficacy in terms of tumor growth control was reported if compared to radiotherapy as a single treatment modality. If capecitabine is used at relatively low doses (1,600–2,000 mg/m2 per day), grade 3 hematological or other toxicity is rare. For these reasons, after a pilot study to establish the safety of the combined therapy protocol, we started a randomized trial comparing treatment with 177Lu-octreotate with and without capecitabine in patients with GEP NETs. Also, attempts to improve the results of this type of therapy may focus on further reducing the radiation absorbed dose to normal tissues and organs, 9 such as the kidneys and bone marrow, or at increasing the receptor density on the tumors, for instance via receptor upregulation. Both strategies may increase the therapeutic window. Intra-arterial treatment in selected patients with a predominant tumor load in the liver has been reported to be safe and effective. McStay et al.used [90Y-DOTA0,Tyr3]lanreotide administered via the hepatic artery (mostly 2 × 1 GBq) to treat 23 patients with NETs, and 3 of these patients showed PR and 12 showed SD. However, 2 of the 3 patients with PR also had had concomitant embolization. Clinical improvement and a decrease in serum tumor markers were observed in 60% of the patients. Limouris et al used [111In-DTPA0] octreotide (6.3 GBq per injection and with a maximum of 12 injections per patient) to treat 17 patients, and 9 of these patients showed CR or PR. Lastly, Kratochwil et al found a fourfold higher uptake after intra arterial administration of [68Ga-DOTA0,Tyr3]octreotide compared with intravenous administration in the same patients. Therefore, in selected patients this type of administration seems advantageous. The use of PRRT as a neoadjuvant treatment has also been advocated in animal studies by Breeman et al. who reported an increased survival in rats treated for 8 days with 177Lu-DOTATATE after infusion of 0.25 × 106 viable CA20948 cells into the portal vein, which mimics liver micro metastases. In humans, the use of PRRT in previously judged inoperable NETs has been described by Kaemmerer et al. in a case report with PRRT used in a neoadjuvant setting before surgery, by Kwekkeboom et al. who reported that PRRT enabled surgery in four patients in a large group of more than 500 patients, by Sowa-Staszczak et al. in two of six patients treated in a neoadjuvant setting, and by Barber et al. who found the same in five.
The case study
The study was on the patient Hiba ,who suffers from adrenal cancer (Phyochromocytoma) a type of endocrine tumor with spread on the spine, pelvis, thorax and thigh parts ,The patient is 31 years old, She has a university degree, and married with no children .Lutetium Peptide receptor radioisotope treatment was performed for her according to the protocol explained below and the result were compared to the chemotherapy which was performed before the PRRT to show the difference and prove the efficiency of our cancer treatment method .
Pheochromocytoma
It is a neuroendocrine tumor of the medulla of the adrenal glands (originating in the chromaffin cells), or adrenal chromaffin tissue that failed to involute after birth, that secretes high amounts of catecholamine, mostly norepinephrine, plus epinephrine to a lesser extent. The term is from Greek phaios “dark”, Chroma “color”, kytos “cell”, -oma “tumor”.

The signs and symptoms of a pheochromocytoma are those of sympathetic nervous system hyperactivity including: 1
- Skin sensations.
- Flank pain.
- Elevated heart rate.
- Elevated blood pressure, including paroxysmal (sporadic, episodic) high blood pressure, which sometimes can be more difficult to detect; another clue to the presence of pheochromocytoma is orthostatic hypotension (a fall in systolic blood pressure greater than 20 mmHg or a fall in diastolic blood pressure greater than 10 mmHg upon standing).
- Palpitations
- Anxiety often resembling that of a panic attack
- Diaphoresis (excessive sweating)
- Headaches – most common symptom.
- Pallor
- Weight loss.
- Elevated blood glucose level (due primarily to catecholamine stimulation of lipolysis (breakdown of stored fat) leading to high levels of free fatty acids and the subsequent inhibition of glucose uptake by muscle cells
Not all patients experience all of the signs and symptoms listed, the most common presentation is headache, excessive sweating, and increased heart rate, with the attack subsiding in less than one hour. Tumors may grow large, but most are smaller than 10 centimeters (4 in). Pheochromocytoma occur most often during young-adult to mid-adult life. Although pheochromocytomas may grow to large size (>3 kg), most weigh <100 g and are <10 cm in diameter, pheochromocytomas are highly vascular. Fewer than 10% of these tumors are malignant, as with several other endocrine tumors, malignancy cannot be determined from the histologic appearance; so precise monitoring and follow up is required for those who develop the disease. It is a tumor that involve the adrenal gland, Adrenal gland consist of the cortex. And the medulla which produce adrenaline the disease is Benin 90% and malignant 10% .But in both cases an increase in the produced adrenaline causes the patient to have hypertension with non-cause, so after some investigation, hormone adrenalin found to be high in kidneys due to the tumor presence. MIBG is a radio tracer that has chemical structure proper to presynaptic adrenaline receptor in adrenal gland, so it is useful to undergo MIBG scan to investigate the presence of the tumor.
Phyochromocytoma usually express large numbers of these receptors, so that it shows intense uptake on MIBG scan, some tumors also shows intense Gallium DOTATATE uptake due to (somatostatin) receptors expression, therefor it shows Gallium uptake on gallium PET-scan and might benefit from the scan to test for the probability of (Lutetium DOTATATE therapy) .
However non differentiated types of tumors do not express the (somatostatin) or MIBG receptors, so that they appear negative on MIBG and Gallium DOTATE scan, so Lutetium therapy is not advised in these cases , on the other hand these non-differentiated tumors shows intense FDG uptake on PET imaging . The previously mentioned symptoms begun with the patient almost four years ago, exactly in 2014, when the patient begun to suffer frequent exhaustion in addition to a permanent feeling of tiredness and constant fatigue with pale skin color associated with her in most times. Doctors advised a magnetic resonance image as soon as possible, however, indeed, the result of the MRI image was a clear tumor in the adrenal gland so the doctors who were in charge suggested the beginning of the tumor treatment and diagnosis work .
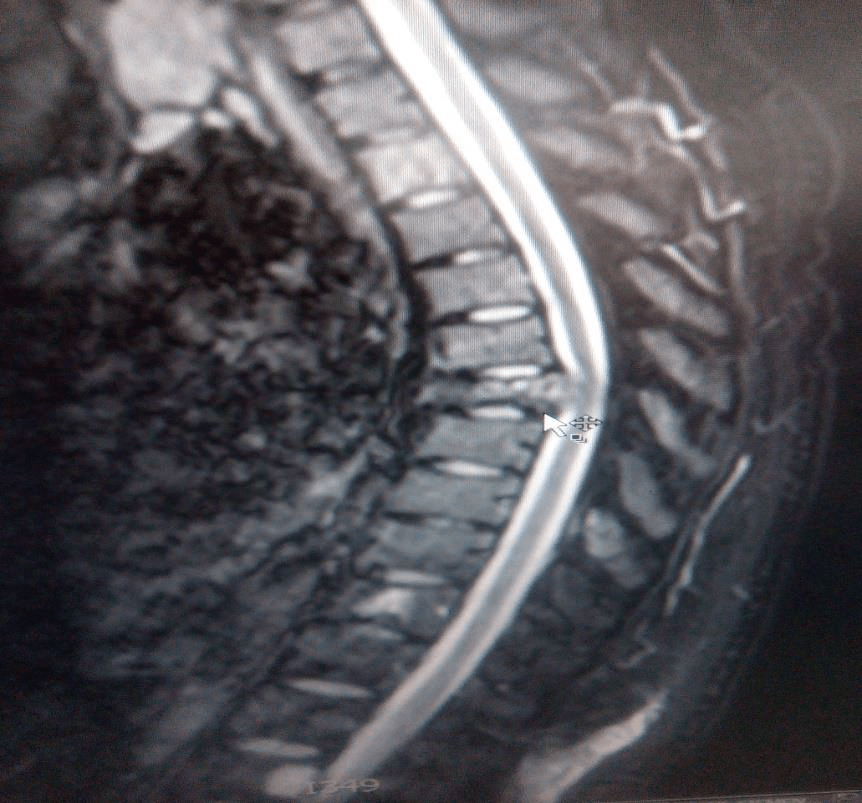
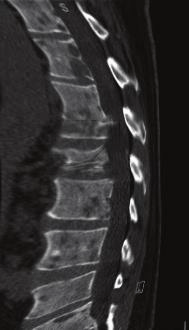
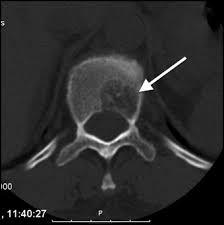
The tests were repeated for the patient again, general and comprehensive blood tests were performed and all agreed to a high levels of adrenal hormones in blood , radiographic images were also done in addition to magnetic resonance images throughout the body, the result of the tests and imaging was for the brain CT scan showed that there was no focal brain lesion and it was a metastatic spread test only, and no shift of midline structure and normal ventricular system, as the lumber spine CT showed that the findings were evidence of multiple bony lytic lesions seen in the lumber spine and sacral bone and no evidence of disc herniation or nerve root compression as showed in figure (7 ), this all tells that all back pains suffered by the patient were due to the presence of the tumor only .
The pelvic MRI showed that there were multiple hyper intense signals seen in the left ischium, that suggested bone metastasis while the abdominal MRI showed that there were a 6.75.3 cm right a adrenal mass lesion which appears isointense with thick wall moreover the neck Ultra sound showed a (97*11 ) mm nodule in the lift lobe with spots of calcification in the thyroid gland enlarged and indicating thyroiditis, after all of those critical signs shown through the previous medical scans , PET-CT scan was advised to insure the presence and the location of the primary lesions and the metasticitc spread , and after undergoing a PET-Ct scan the result showed a hypermetabolic and mostly malignant right adrenal mass with bone metastasis .
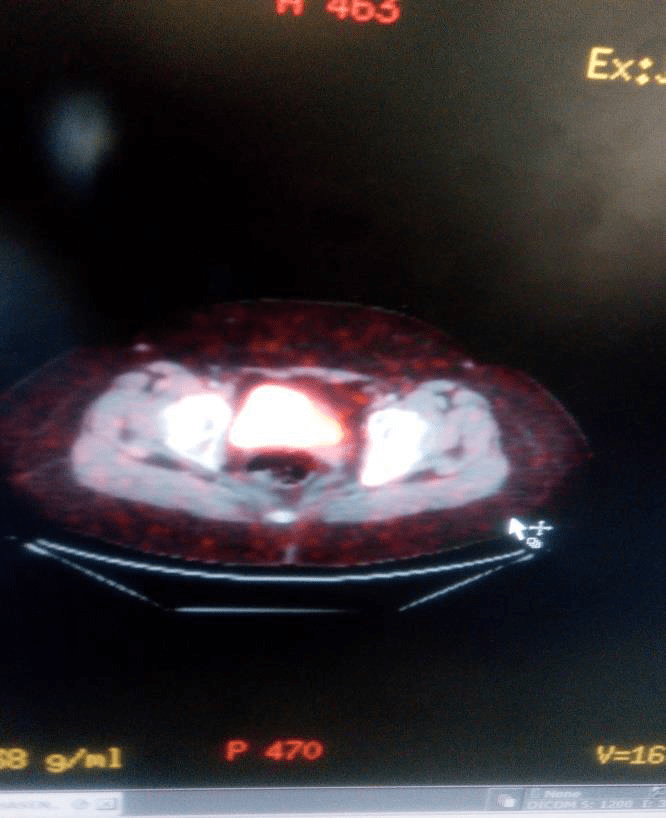
For evaluation of kidneys functioning TC99 MAG3 dynamic renal scan with (Lasix) was performed and both kidneys were good functioning with good perfusion and excretory response.
The first decision was a surgery, to completely eradicate the adrenal gland and the tumor associated with it, and after the operation doctors suggested the chemotherapy to get rid of any remnants of the tumor in the place of surgery and the distant metastasis.(16).
Twenty doses were given along two years, during the chemotherapy trip, the patient was given a boosted injection of limestone to the bones as well as medications to maintain blood pressure at regular levels, after the operation. Her high blood pressure was observed. In addition to many painkillers, such as Morphine and Tramal , As for the effect of chemotherapy and as the patient tells, there have been a lot of fears and a great deal of post-treatment psychological aspects, indeed, while taking doses one by one the symptoms of stress and fatigue began to appear on the patient and her hair was falling, with a bad emotional situation and weak food appetite and general feeling of weakness associated with a long night pains in various areas of the body especially the spine and was often forced to visit the hospital at night to take powerful intravenous analgesics, this all led to a low evaluation of life quality as the patient was questioned through a standard chemotherapy effects questioner of the JRMS which showed reduced value of life for the patient and reduced patient’s emotional comfort showing a very bad chemotherapy side effects on the psychological .(16).
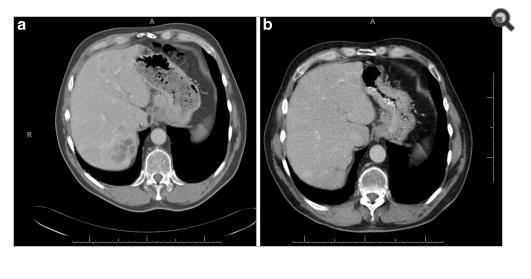
Associated with all of the before the tumor remission was so slow or to be clear no remission occurred at all and no valuable improvement appeared as showed in the figures below
For Pet-Ct images taken for the patient pre and after the chemotherapy which shows no significant changes in the tumor distribution or size after chemotherapy.
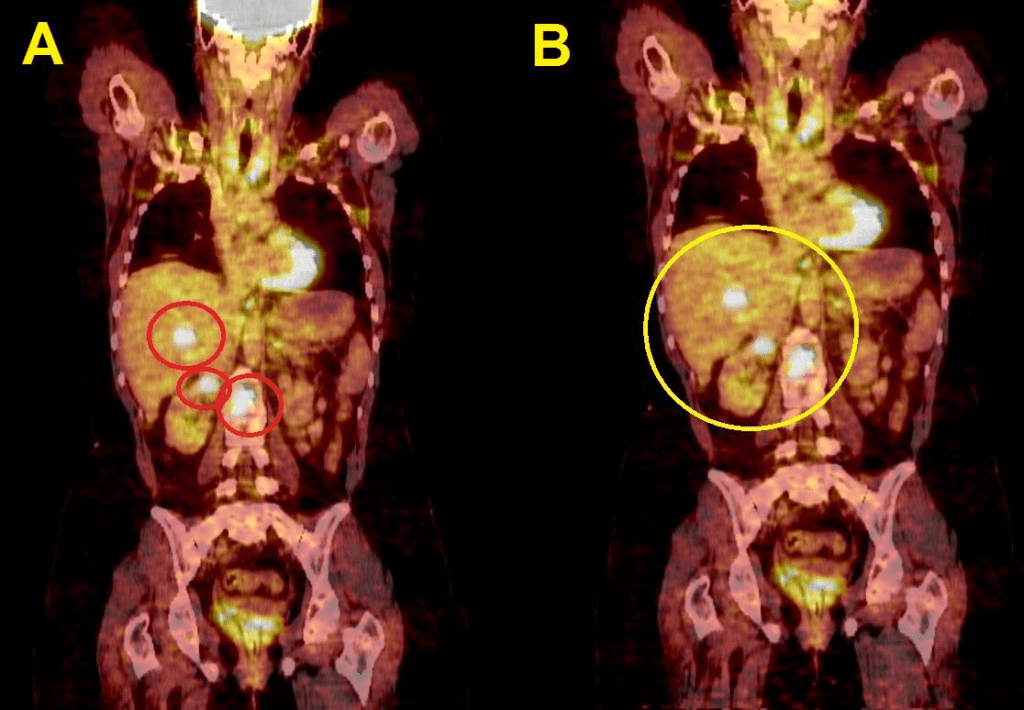
nificant difference from the previous scan in 2004. (16- Jordanian medical services, PACS system and database store center).
Discussing these unwelcomed results of Chemotherapy the need for better treatment technique appeared, and a Gallium DOTATE scan was done to check for PRRT possibility, and since the tumor showed intense Gallium DOTATE on Gallium scan due to somatostatin receptor express by the tumor, we expect good Lutetium DOTATATE uptake by that tumor and therefor good responses to this type of therapy.(9).
Lutetium and DOTATATE interaction with tumor cells
The lutetium DOTATATE binds to the (somatostatin) receptors on the cell wall of tumor cells and the is transported inside the Cell where the Lutetium decays and emits beta particles, these beta particles causes highly damage to the DNA molecule which causes the destruction of the tumor cells . (3).
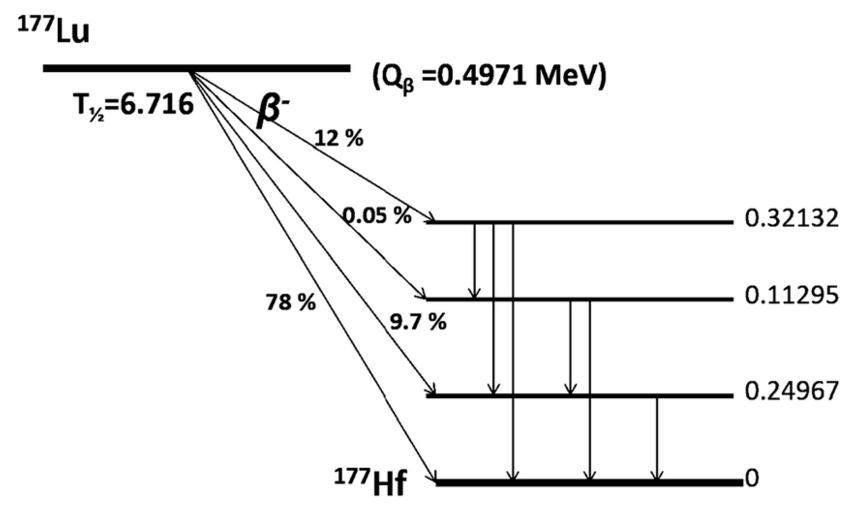
Lutetium physical characteristics 177Lutetium (177Lu) has gained popularity as the therapeutic radionuclide of choice due to its desirable physical properties. Ideally, the emission characteristics of a therapeutic radionuclide should match the lesion size and volume to be treated to ideally focus energy within the tumor rather than in the tissue surrounding the lesion. 177Lu is a medium‐energy β‐emitter (490 keV) with a maximum energy of 0.5 MeV and a maximal tissue penetration of <2 mm. The shorter β‐range of 177Lu provides better irradiation of small tumors, in contrast to the longer β‐range of 90Y and as it is shown in the figure below 177Lu is a reactor produced radionuclide that emits low‐energy γ‐rays at 208 and 113 keV with 10% and 6% abundance respectively. The gamma emission from 177Lu allows for gamma imaging and consequently the collection of information pertaining to tumor localization and dosimetry, furthermore, 177Lu has a relatively long physical half‐life of 6.73 days. So these are a very useful physical properties that allow for the delivery of high doses of 177Lu to cancer cells and allow any dosimetry or diagnostic imaging after treatment.(3) .
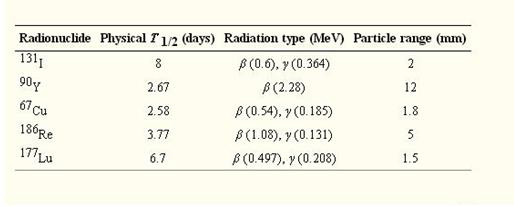
Table 3: Table of the radionuclides used in targeted therapy showing emission energies and ranges. (7).
Injection protocol
used in the JRMS nuclear medicine center takes care about kidney protection, as the patient is injected initially for half an hour with two types of amino acids, Arginine and Lysine amino acids in a solution with normal saline ( H2O and NaCl 8%).
To clarify the point ,those amino acids can bind to the great number of somatostatin receptors in the cortex of the kidney which may bind the most to Lutetium DOTATAE if no kidney protection is used , the patient is then injected with radioactive lutetium with a dose of 200 mCi as a solution (Lutetium chloride DOTATAE) , 200 mci is not very high because most of the dose deposited will be deposited locally in the tumor rejoin without causing any dose delivery in the surrounding normal tissue, and this is clear from the very short range of the beta particles emitted by Lutetium 177 and the very small fractions of gamma emission from the lutetium nuclei.
Another dose of amino acids is delivered for another half an hour, then the patient returns home having an isolation of two days at least to protect the children or pregnant women around from the Gamma emission of the radioactive Lutetium as doses accumulated at distant of less than two meters around the patient along the two days after treatment were less than 100 micro severts according to our study for the patient for three treatment cycles in the isolation room. Patient must undergo a gamma camera scan in the next day and that is done as a pre and after ablation imaging to insure the therapeutic changes that Lutetium caused.
Scan procedure
In the day of scan which is the next day after the injection, patient should go to the toilet and empty the bladder and then the gamma camera is used to have a whole body scan that can serve to verify the following things:
Check the distribution.
Verify the activity.
Post ablation or pre ablation imaging.
Because the gamma radiation from the lutetium is very low as shown in the decay scheme, the imaging technician is forced to increase the test time to the maximum time possible as shown in the figure (12), the scan must go with a very slow speed to collect larger number of count at least up to 800 counts per scan.

Medium collimator is used as the 208 Kev is a medium range energy. With peaking controls that uses two Energy windows for 208 Kev with a ratio of 10.3% as a high peak and 113 Kev with a ratio of 6.2% for the low peak with 20% windows for both as shown in the figure (13).
.

Another imaging parameters are important for the image quality, as using a matrix size of 256*1024, with 1X zooming, the energy preset to Lu177, and we choose the both detectors for good counts, as showed in the figure below.
The image matrix should be 256×1024 to insure good resolution of the image with no more than 1 degree of zooming to maintain image resolution as best possible.

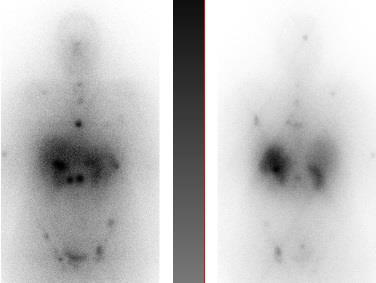
Finally we use a point source of Lu177 with a very low activity less than 50 micro curie to adjust the camera before imaging, then we start whole body scan, with scan speed of 5cm/min the average 170cm length patient remains on the table about 35 minutes .The final image of our patient was as follows in the figure and there we all can see the spreaded metastasis.
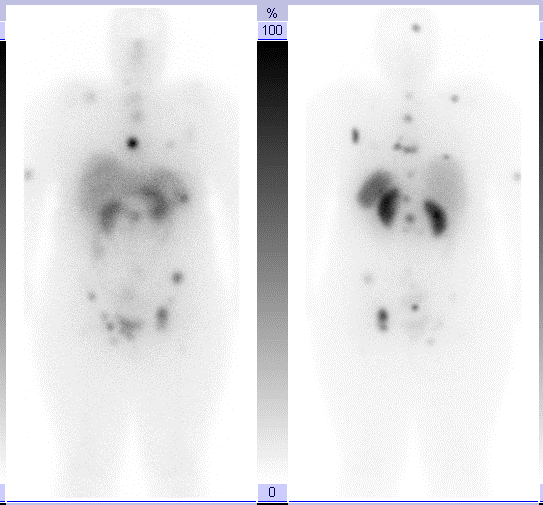
The first treatment was done and a pre ablation scan was performed and showed a wide spread metastases. Six months after this image the patient was imaged again after the repetition of the previous Lu177DOTATE therapy as a second cycle treatment. The next day of the injection and here a great difference shows in the image as a great benefit and good remission in the
metastatic malignancy due to the use of Lu177DOTATE therapy which did not happen with any other treatment technique used with the patient specially the chemotherapy used before.
Figure17: Great PR appears as a result of the 177Lutitum DOTATAT radionuclide therapy after 6 months of the first cycle (16- Jordanian medical services, PACS system and database store center).
Conclusion
Treatment with radiolabeled somatostatin analogues is a promising new tool in the management of patients with inoperable or metastasized neuroendocrine tumors, treatment with 177Lu-labelled somatostatin analogues that have been used in PRRT may lead to symptomatic improvement, and the results obtained with 177Lu-DOTATATE are very encouraging in terms of tumor regression.
Dosimetry studies with 177Lu-DOTATATE as well as the limited side effects after 177Lu-DOTATATE treatment suggest that there is room for more cycles of 177Lu-DOTATATE than currently used, also, if kidney protective agents are used, the side effects of PRRT are few and mild, and less than from 90Y-DOTATOC. CR, PR or MR may be achieved in almost higher number of patients and the duration of the therapy response is more than 40 months. The patients’ self-assessed QoL increases significantly after treatment with 177Lu-DOTATATE. Lastly, compared to other types of tumor treatments, the patient treated with 177Lu-DOTATATE show an increase in overall tumor control which was significantly clear in the post ablation diagnostic images.
If more widespread use of PRRT can be guaranteed, such therapy may well become the therapy of first choice in patients with metastasized or inoperable NETs.
References
- Rinke A, Muller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27(28).
- Krenning EP, Bakker WH, Breeman WA, Koper JW, Kooij PP, Ausema L, et al. Localisation of endocrine-related tumours with radioiodinated analogue of somatostatin.. 1989.
- Balon HR, Goldsmith SJ, Siegel BA, Silberstein EB, Krenning EP, Lang O, et al. Procedure guideline for somatostatin receptor scintigraphy with (111)In-pentetreotide. J Nucl Med. 2001.
- Valkema R, De Jong M, Bakker WH, Breeman WA, Kooij PP, Lugtenburg PJ, et al. Phase I study of peptide receptor radionuclide therapy with [In-DTPA]octreotide: the Rotterdam experience. Semin Nucl Med. 2002. (2).
- Anthony LB, Woltering EA, Espenan GD, Cronin MD, Maloney TJ, McCarthy KE. Indium-111-pentetreotide prolongs survival in gastroenteropancreatic malignancies. Semin Nucl Med. 2002;32(2).
- Walrand S, Flux GD, Konijnenberg MW, Valkema R, Krenning EP, Lhommel R, et al. Dosimetry of yttrium-labelled radiopharmaceuticals for internal therapy: 86Y or 90Y imaging? Eur J Nucl Med Mol Imaging. 2011.
- O’Mara RE, McAfee JG, Subramanian G. Rare earth nuclides as potential agents for skeletal imaging. J Nucl Med. 1969..
8.Bard DR, Knight CG, Page-Thomas DP. Effect of the intra-articular injection of lutetium-177 in chelator liposomes on the progress of an experimental arthritis in rabbits. Clin Exp Rheumatol. 1985. - De Jong M, Breeman WA, Valkema R, Bernard BF, Krenning EP. Combination radionuclide therapy using 177Lu- and 90Y-labeled somatostatin analogs. J Nucl Med. 2005.
25 - Sward C, Bernhardt P, Johanson V, Schmitt A, Ahlman H, Stridsberg M, et al. Comparison of [177Lu-DOTA0,Tyr3]-octreotate and [177Lu-DOTA0,Tyr3]-octreotide for receptor-mediated radiation therapy of the xenografted human midgut carcinoid tumor GOT1. Cancer Biother Radiopharm. 2008.
- Wehrmann C, Senftleben S, Zachert C, Muller D, Baum RP. Results of individual patient dosimetry in peptide receptor radionuclide therapy with 177Lu DOTA-TATE and 177Lu DOTA-NOC. Cancer Biother Radiopharm. 2007.
- Frilling A, Weber F, Saner F, Bockisch A, Hofmann M, Mueller-Brand J, et al. Treatment with (90)Y- and (177)Lu-DOTATOC in patients with metastatic neuroendocrine tumors. Surgery .2017.
- Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008.
- Reubi JC, Waser B, Schaer JC, Laissue JA. Somatostatin receptor sst1-sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur J Nucl Med. 2001.
- Kwekkeboom DJ, Bakker WH, Kooij PP, Konijnenberg MW, Srinivasan A, Erion JL, et al. [177Lu-DOTAOTyr3]octreotate: comparison with [111In-DTPAo]octreotide in patients. Eur J Nucl Med. 2001.
16- Jordanian medical services, PACS system and database store center.